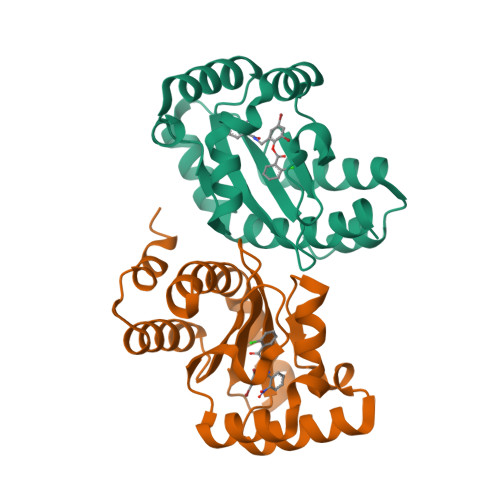
Molecular basis for the recognition of structurally distinct autoinducer mimics by the Pseudomonas aeruginosa LasR quorum-sensing signaling receptor.
Zou, Y., Nair, S.K.(2009) Chem Biol 16: 961-970
- PubMed: 19778724
- DOI: https://doi.org/10.1016/j.chembiol.2009.09.001
- Primary Citation of Related Structures:
3IX4, 3IX8, 3JPU - PubMed Abstract:
The human pathogen Pseudomonas aeruginosa coordinates the expression of virulence factors using quorum sensing, a signaling cascade triggered by the activation of signal receptors by small-molecule autoinducers. These homoserine lactone autoinducers stabilize their cognate receptors and activate their functions as transcription factors. Because quorum sensing regulates the progression of infection and host immune resistance, significant efforts have been devoted toward the identification of small molecules that disrupt this process. Screening efforts have identified a class of triphenyl compounds that are structurally distinct from the homoserine lactone autoinducer, yet interact specifically and potently with LasR receptor to modulate quorum sensing (Muh et al., 2006a). Here we present the high-resolution crystal structures of the ligand binding domain of LasR in complex with the autoinducer N-3-oxo-dodecanoyl homoserine lactone (1.4 A resolution), and with the triphenyl mimics TP-1, TP-3, and TP-4 (to between 1.8 A and 2.3 A resolution). These crystal structures provide a molecular rationale for understanding how chemically distinct compounds can be accommodated by a highly selective receptor, and provide the framework for the development of novel quorum-sensing regulators, utilizing the triphenyl scaffold.
Organizational Affiliation:
Department of Biochemistry, University of Illinois at Urbana-Champaign, 600 S. Mathews Avenue, Urbana, IL 61801, USA.